cuo + nh3 observation CuO NH3
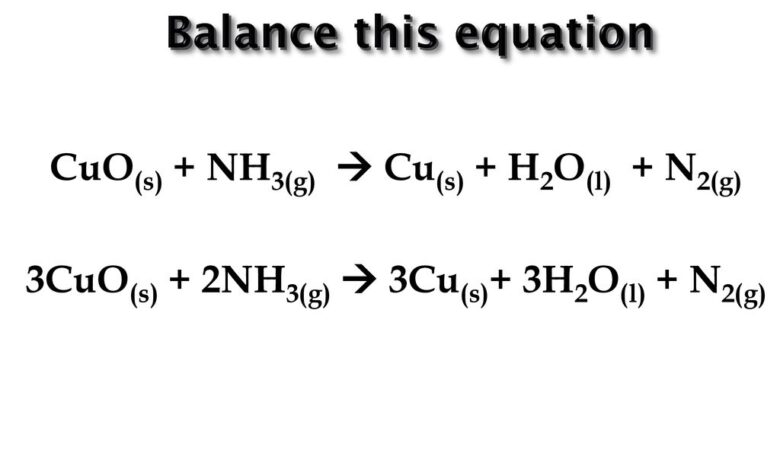
adding concentrated HCl (aq) to CuCl. CuO + H2 → Cu + H2O. NH3 +CuO = Chất rắn A + Hỗn hợp khí B. Nhờ các bạn giải giúp Cho lượng khí NH3 đi từ từ qua ống sứ chứa 3,2g CuO nung nóng đến khi phản ứng xảy ra hoàn toàn ; thu được rắn A và 1 hỗn hợp khí B. Chất rắn A phản ứng vừa đủ với 20 ml HCl 1M. Allow about 10 minutes for this dùng thử. a.Viết ptpu. Please register to post comments. This red-coloured solid is a component of some antifouling paints. slowly add ammonia until a pale blue precipitate forms, add more ammonia until a deep blue solution forms, add sulfuric acid until the pale blue precipitate reforms, continue to add sulfuric acid until the original clear, pale blue solution reforms. Two skeletal schemes under low or high temperature conditions are proposed. Copyright © 2021 Elsevier B.V. or its licensors or contributors. The university shall not be liable for any special, direct, indirect, incidental, or consequential damages of any kind whatsoever (including, without limitation, attorney’s fees) in any way due to, resulting from, or arising in connection with the use of or inability to use the website site or the content. The custom demos section of the website is used by UO chemistry instructors to schedule demonstrations that are not listed in the database. and Move the slider left to right to see the impact of covid-19 in China: compare 2020 with the average of the 3 previous years during and after the lockdown. The copper-ammonia complex ion is a deep purple,which makes … The Calitha – GOLD engine (c#) (Made it … N 2 O 4 is a diamagnetic so it is colourless. We use cookies to help provide and enhance our service and tailor content and ads. UO Libraries Interactive Media Group. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, … reducing [CuCl4]2- with Cu or So2. So oxidation number of nitrogen is reduced from +5 to +2 while oxidation number of copper is increased from 0 to +2. This dùng thử is a good illustration of Le Chatelier’s principle and of complex ion formation. The simplest way of representing this sytem is: CuCl 4 2-(green) + 4 H 2 O <==> Cu(H 2 O) 4 2+ (blue) + 4 Cl-.This equilibrium is probably the best one to present for a discussion of Le Chatelier’s principle in an intro Gen Chem class, but the system is probably more complicated, possibly involving the formation of mixed complexes. Amount of added Cu at beginning will be same after the reaction. Copper oxide react with ammonia and water CuO + 4NH 3 + H 2 O → [Cu (NH 3) 4 ] (OH) 2 [ Check the balance ] Copper oxide react with ammonia and water to produce hydroxide diamminecopper (II). Copper (I) chloride is prepared by? Instructions. The major problem of CuO-based SCR catalysts is well elucidated. Direct link to this balanced equation: Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click ‘Balance’. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Start studying Copper lab observations and equations. CuO (s) + H2 (g) → Cu(s) + H2O (g) a) Name the oxidized substance. It burns with yellowish-green flame producing water vapour and nitrogen. What observations can be made? In an excess of NH3(aq), Cu2+ ion forms a deep blue complex ion, Cu(NH3)42+, which has a formation constant Kf = 5.6 1011. If you heat the made solution of copper sulphate, the water will evaporate and you will be left with white anhydrous copper sulphate crystals. What a great software product!) This reaction uses hazardous substances – see below. CuO: 3: 79.5454: N 2: 1: 28.0134: Cu: 3: 63.546: H 2 O: 3: 18.01528: Units: molar mass – g/mol, weight – g. Please tell about this free chemistry software to your friends! Unformatted text preview: Lab 6: Chemical Reactions Data Tables Name: _____ Data Tables Section I: Reacting Mg with HCl Solution: Your Observations Appearance of the Mg A piece of Mg ribbon was placed in the solution.The Mg is a small shiny piece. what colour and state is CuCl. Calculate the Cu2+ concentration in a solution prepared by adding 4.8 10-3 mol of CuSO4 to 0.500 L of 0.32 The university further disclaims all responsibility for any loss, injury, claim, liability, or damage of any kind resulting from, arising out or or any way related to (a) any errors in or omissions from this website site and the content, including but not limited to technical inaccuracies and typographical errors, or (b) your use of this website site and the information contained in this website site…the university shall not be liable for any loss, injury, claim, liability, or damage of any kind resulting from your use of the website site. Avoid breathing ammonia vapors. The answer will appear below The nitridation degree of CuO surface is usually not too high. The formation of [Cu(NH3)4(H2O)2]^2+ complex results in a decrease in [Cu^2+] which then cause the position of equilibrium of (1) to shift right so as to form back some Cu^2+. Cu behaves as a catalyst and NH 3 will be oxidized to NO. white and solid. © Copyright 2012 Tin nhắn hộp thư online: 500 mL three-necked round-bottom flask containing 200 mL of 0.1M copper(II) chloride dihydrate in water and a magnetic stir bar, 24/40 vertical ground glass joints. Abstract Understanding the oxidation of ammonia (NH 3) over CuO surface and then the formation routes of N 2 and NO x is rather crucial to provide a favorable direction for the rational thiết kế of high-performance Cu-based oxygen carriers in chemical looping combustion (CLC) and CuO NH3-containing catalysts in selective catalytic reduction (SCR). Reaction Type. addition funnel containing 150 ml of concentrated ammonia solution (ammonium hydroxide), 24/40 ground glass joints. ScienceDirect ® is a registered trademark of Elsevier B.V. ScienceDirect ® is a registered trademark of Elsevier B.V. To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. N2(g) + 3H2O(l) + 3Cu(s) Complete The Statements Below. Finally, the effects of O2 and H2O on the fate of nitrogen during heterogeneous reactions have also been determined. Copper is an essential mineral that plays a key role in many physiological processes, including angiogenesis, skin generation and expression and stabilization of skin proteins. Nitric oxide is a colorless gas and oxidation number of nitrogen is +2. Click hereto get an answer to your question ️ In the reaction, 3CuO + 2NH3 N2 + 3H2O + 3Cu the change of NH3 to N2 involves: b) Name the reduced substance. Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu 2 O. Consider the following statements and arrange in the order of true/false as given in the codes. So the substances getting reduced is CuO. As more ammonia is added the insoluble Cu (OH)2 will dissolve as [Cu (NH3)4]2+ is formed. NH3 + O2 = NO + H2O – Chemical Equation Balancer. By continuing you agree to the use of cookies. Therefore Cu(OH)2 ppt dissolves in excess NH3(aq) to give a dark blue solution. Randy Sullivan, University of Oregon The university expressly disclaims all warranties, including the warranties of merchantability, fitness for a particular purpose and non-infringement.



